A place for everything
The correct use of dental materials is essential to successful outcomes for the patient. Here, Steve Bonsor begins a series of articles on dental materials, beginning with an examination of pre-placement factors
Dental material manufacturing companies go to great lengths and spend large amounts of money developing dental materials which, if used correctly, will yield predictable clinical results. Unfortunately, there are many factors outwith the control of the manufacturer which may subsequently contribute to failure of the material, thereby decreasing its longevity.
This article, the first of two on the use and abuse of dental materials, explores the potential problems which can arise even before the dental material has been placed in the mouth and offers practical advice on how these factors may be circumvented to optimise clinical success.
Cause of failure
In 1990, Mjör et al. published a paper examining the cause of failure when working with dental materials clinically. He found that 88 per cent of all failures were technique related, with the balance being material failures. Operator (or more correctly, dental team) variability is one of the commonest causes of failures.
Although mechanical mixing may eliminate many potential errors, it is not a panacea and not without shortcomings. For this reason, compules have evolved
Steve Bonsor
During the manufacturing process, the manufacturer ensures that each product is supplied in perfect condition. However, when the product arrives in the clinic, there is much potential for the material to be compromised by incorrect handling.
Importance of following instructions
Long before the material is manipulated at the chairside, there are various factors which could compromise its clinical success. Very often, the first thing that happens when a new product arrives in the clinic is that the Directions For Use (DFU) supplied with each product is often discarded (Fig 1).
In dentistry, and not dissimilar to the assembly of flat pack furniture, there is a popular misconception that the DFU should only be referred to when there is a problem which the operator cannot solve. It is critically important that the DFU is read carefully prior to use and followed fastidiously.
- Figure 1 The Directions For Use booklet supplied with each product should not be discarded but retained for reference by the dental team
- Figure 2 A ring binder file containing the DFUs of all the materials used in the clinic inserted into poly-pockets
- Figure 3 Metering device to monitor stock room temperature and humidity
- Figure 4 Drops of water condensing on a chilled glass slab. Incorporation of this water into the powder/liquid mix will affect the final properties of the cement
- Figure 5 Rubber dam isolation to control the moisture, both in vapour and liquid forms, during a bonding procedure
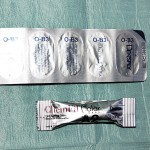
- Figure 6 An example of a compomer material (Dyract Extra, Dentsply) presented in foil blister packs to prevent moisture from the air contaminating the material, and excluding light
- Figure 7 An agate spatula which should be used to hand mix glass ionomer cements
- Figure 8 An example of a capsule containing a glass ionomer cement (ChemFil Supreme, Dentsply)
- Figure 9 The contents of a capsule: 1. Plastic clip 2. Pillow 3. Powder 4. Access hole 5. Outer casing 6. Plunger
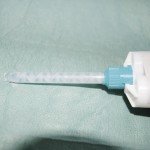
- Figure 10 Compules containing a resin-based composite material (Miris 2, Coltene Whaledent)
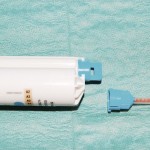
- Figure 11 A cartridge mixing system with its spiral delivery tube prior to attachment (Protemp 4, 3M ESPE)
- Figure 12 Cartridge delivery system containing a bite registration material with mixing tip in situ to prevent cross-contamination between the two tubes leading to premature set (Memosil 2, Heraeus Kulzer)
Dental materials do evolve with time and the manufacturer may make slight modifications to products. Therefore, the DFU will consequently change. The dental team should be cognisant of this and adapt accordingly, otherwise the material may be used inappropriately, resulting in decreased clinical performance.
The member of staff responsible for ordering and stock control could be delegated to check every new pack of material for changes in the DFU upon arrival in the clinic and disseminate these to the rest of the team if necessary. Many clinics collate the DFUs in a materials file for easy reference (Fig 2). Only one set of instructions per product need be kept and this file can be kept centrally where all staff can readily access. The file is also useful for COSHH (Control of Substances Hazardous to Health) purposes.
Effect of temperature and humidity on materials
It is important that the stock room environment is carefully controlled so that materials are stored in optimum conditions and do not degrade prematurely. Unfortunately, this is very often overlooked. It is desirable for the material store to have an ambient temperature and humidity. This may be monitored easily by purchasing an inexpensive gauge from a hardware store (Fig 3).
Temperature has a major influence on materials during storage with extremes having detrimental effects. In a hot environment, materials may prematurely age even before they reach their ‘use by’ date. Furthermore, increased temperature accelerates the setting reaction of the material leading to premature set. As the temperature tends to be higher in the dental surgery itself, it is therefore sensible to keep a minimum amount of material in this room.
At the other extreme, too cold conditions decrease setting time and increase working time. The viscosity of some materials (such as some elastomeric impression materials) may also increase at lower temperature, making them more difficult to mix. Many impression materials are now mixed in a machine and, if the product is not at the correct temperature, its increased viscosity may place undue stresses on the pistons of the machine, potentially inflicting damage.
When mixing cements, if the temperature of the glass slab is reduced to below the dew point (that is the temperature that air must be cooled for water vapour to condense into liquid) then beads of moisture will form on the surface of the slab (Fig 4). This water will then become incorporated into the cement mix, so reducing the powder to liquid ratio and producing a weakened cement.
However, a lower temperature may be beneficial as some materials such as silane coupling agents and whitening agents should be kept in the refrigerator to prolong their shelf life.
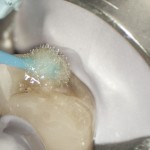
Humidity also has an effect on setting time with increased humidity causing premature set. Furthermore, moisture inclusion into the material may be detrimental and is most significant during the mixing phase. An air conditioning unit may be installed in the surgery to maintain a constant temperature and humidity. Rubber dam is an invaluable tool to provide an environment with a lower humidity compared to exhaled air. This is most critical when a bonding procedure is being carried out using hydrophobic materials such as resin composite (Fig 5).
Importance of product packaging
To ensure that each product arrives in an optimal condition, its packaging is carefully designed and presented. The packaging may serve to protect against light and prevents contamination by moisture in the case of photolytic and hydrophobic materials respectively (Fig 6).
For example, as water is required to complete their setting reaction, any uptake of moisture will cause compomers to set prematurely. It is therefore strongly advised that materials are left in their original packaging until ready for use. The manufacturer’s instructions should also be followed with respect to storage, e.g. keep in the refrigerator, dark etc.
Each dental material has an expiry date and this should be checked prior to use. If this date has passed then the material should be discarded as the catalyst may have degraded and undesirably retarding the set of the product.
Factors affecting mixing
Powders should always be shaken prior to use as settling and compaction occurs over time, so more powder will be dispensed, increasing the powder to liquid ratio. The constituents of the powder may also separate during storage and shaking the bottle will mix the contents thoroughly. Similarly, bottles of liquid should be shaken to ensure a homogeneous solution.
Mixing by hand involves a mixing slab or pad made of glass, silicone or paper and the correct spatula which is compatible with the material being mixed. They must then be used together in the correct manner. The choice of mixing pad will depend on the material being mixed. It is recommended that zinc phosphate is mixed on a chilled glass slab to dissipate the heat produced during its exothermic setting reaction as well as increasing its working time. There are advantages and disadvantages to the other pads.
A spatula appropriate for the material being mixed should be selected. For example, a metal spatula should not be used to mix glass ionomer cement as the abrasive glass may abrade the metal of the instrument during mixing and incorporating it into the mixed material, instead an agate spatula should be used (Fig 7).
Finally, the material should be mixed as per the manufacturer’s instructions. Some resin modified glass ionomer cements presented in powder and liquid form rely on micro-capsular technology. This is similar to the ‘scratch and sniff’ panels used in lifestyle magazines to demonstrate perfumes and aftershave scents. During mixing, the shell of the microcapsule must be broken down to allow the active ingredients to be released from the microcapsule to react with the liquid and this will only occur with proper spatulation.
On occasion, when the clinic is running low of a powder, there may be a temptation to use another similar product to compensate, for example using zinc oxide powders interchangeably. This is a very dangerous strategy as often the chemicals contained in powders vary and, therefore, the material at best will not function as intended and at worse may not set. It is, therefore, good advice never to mix dissimilar or unrelated materials.
Encapsulated cements and mechanical mixing
The presentation of the product has a significant effect on the way the material may be mistreated. While hand mixed materials affords the operator more control to vary proportions, materials that are provided as components which the user must mix are most at risk as the potential for error is very high.
Furthermore, it can be difficult to dispense components in the correct proportion of each and difficult to mix a much smaller volume of one with a large volume of the second and ensure even distribution of the two components. To overcome some of the problems with hand mixed materials, manufacturers offer some powder and liquid products in the form of capsules (Fig 8).
These containers hold the active ingredients in separate compartments until activation (Fig 9). The capsule then becomes the mixing chamber and delivery vehicle. As with hand mixed powders, it is important that the capsule is shaken prior to use to ensure that all of the constituents of the powder are thoroughly mixed.
Pressure must also be applied to the capsule for two seconds so that all of the liquid can be expressed from the pillow to yield the correct powder to liquid ratio. While the desired mix may be more consistently produced, there are some limitations with capsules. It is difficult for manufacturers to provide a precise dose of powder and metering of small volumes of liquid to fill the pillow produces some variation.
Additionally, a small nozzle, which may be the most appropriate size for precise delivery into the cavity, may not be able to physically extrude (more viscous) material with a higher powder to liquid ratio.
The dental team should be aware that different mechanical mixing machines may exhibit different types of movement, such as a figure of eight oscillation or a rotatory movement. The amount of energy imparted to the material contained within the capsule differs due to the throw of the mixing arm and the speed of oscillation. The machine should be set to the correct speed and movement applicable to the product being mixed.
The correct mixing time should also be carefully adhered to. For example, too short a mixing time will realise an incoherent mass with the powder and liquid inadequately mixed. Too long a mixing time will produce a reduced working time and accelerated set. There may also be a reduction in the mechanical properties of the material in some cases. It sounds obvious but the correct time will produce the correct mix and this information may be gleaned from the DFU.
Light-cured product evolution
Although mechanical mixing may eliminate many potential errors, it is not a panacea and not without shortcomings. For this reason, compules (also known as Tips or Cavifils depending on the manufacturer) have evolved. These are small cylindrical containers with a delivery tip and plunger and contain all the active ingredients already mixed together by the manufacturer into an injectable paste (Fig 10).
This is advantageous for many reasons. Firstly, the proportioning of the ingredients and the mixing of the materials are optimised. Secondly, the risk of incorrect mixing is reduced as the components are already blended together, and, thirdly, the product should be (almost) free of air voids. These materials require an external energy source (such as visible light) to initiate the setting reaction.
Cartridges
The other modern method for the mixing and delivery of (impression and bite registration) products are paste-paste systems supplied in cartridges. A spiral tube with a varying number of helical turns (the number depending on the viscosity of the material) which both mixes and delivers the paste is attached to the cartridge containing the base and catalyst (Fig 11).
The material may be conveniently extruded at the site of use. Partly used cartridges should be stored with the previous mixing tip in situ (Fig 12). The original cap should be discarded after the product has been opened and never replaced, as base and catalyst retained at the orifices of the tubes may come into contact, so setting and occluding the exit ports. The set material will also form a seal preventing contamination from the environment.
Some impression materials are incompatible with certain mixing machines. This is a clever marketing ploy employed by some manufacturers to ensure that only their own products can be used in the machine to protect their commercial interests. Without stating the obvious, the dental team should ensure that any new product is compatible with the machine in the clinic.
Conclusions
As has been illustrated in this article, there are many pitfalls into which the dental team may fall when handling dental materials, even before they have been placed intra-orally. The most significant is operator variability which accounts for the commonest causes of failures.
This is despite the efforts of dental manufacturers who have attempted to produce materials which are as foolproof as possible. It is therefore imperative that the dental team reads and follows the DFU to ensure optimum performance and treatment outcome.
The author
Mr Steve Bonsor graduated from the University of Edinburgh in 1992 and in 2008 gained a MSc in Postgraduate Dental Studies from the University of Bristol. From 1997 until 2006 Steve was a part-time clinical teacher at Dundee Dental Hospital and School and honorary clinical teacher at the University of Dundee in the sections of operative dentistry, fixed prosthodontics, endodontology and integrated oral care.
He currently holds appointments at the University of Edinburgh, as an online tutor on the MSc in Primary Dental Care programme and at the University of Aberdeen as honorary clinical senior lecturer leading the applied dental materials teaching at Aberdeen Dental School.
As well as lecturing throughout the UK, Steve is actively involved in research, having published original research articles in peer-reviewed journals. His main research areas are photo-activated disinfection and the clinical performance of dental materials.









Comments are closed here.